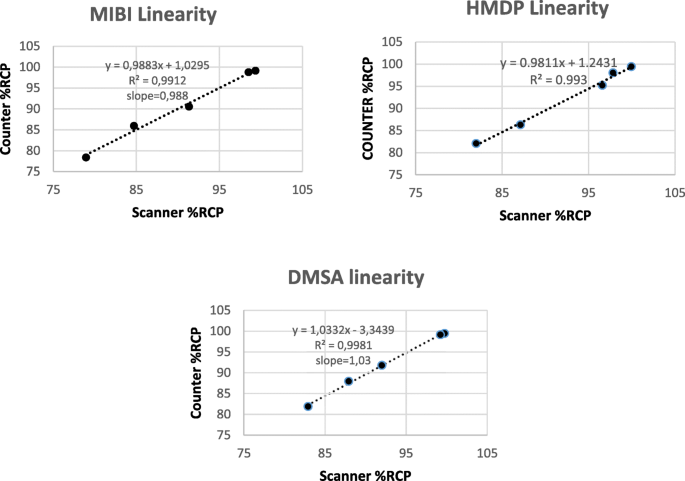
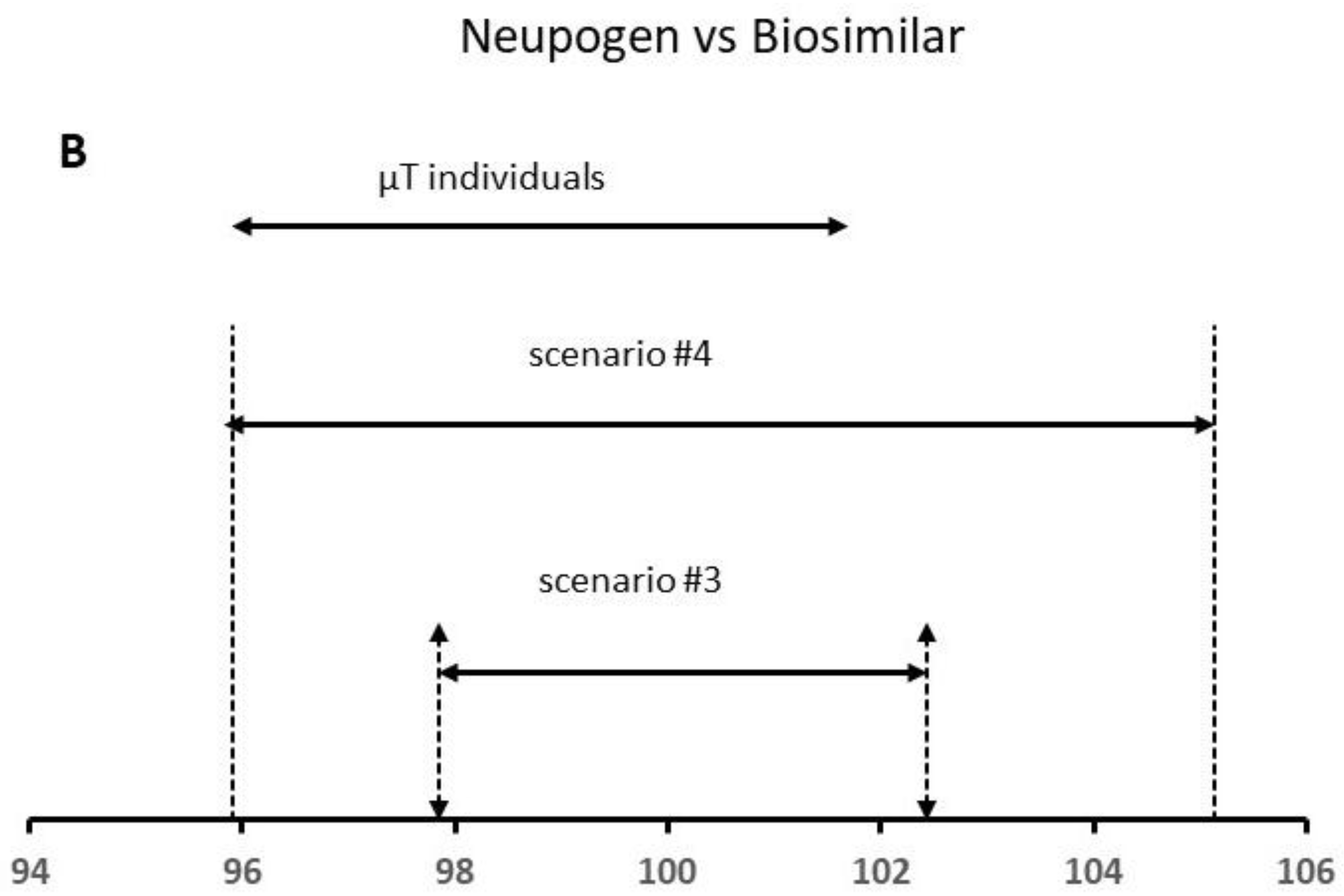
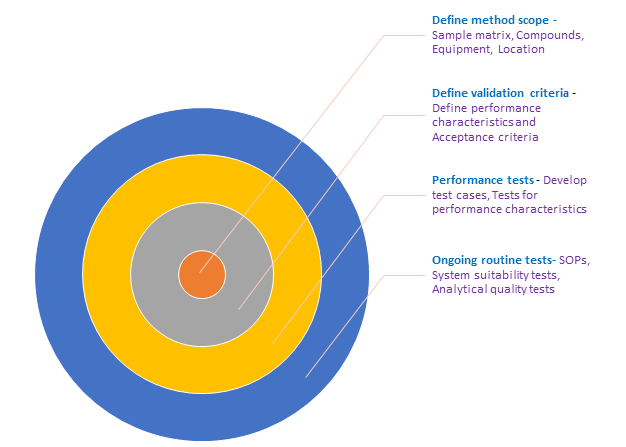
O administrador coleta informações Ich Q2 R1 Validation Of Analytical Procedures Text And Methodology.
27 october 1994 q2. Q2 approval by the steering committee under step 2and release for public consultation.
Q14 analytical procedure development guideline.

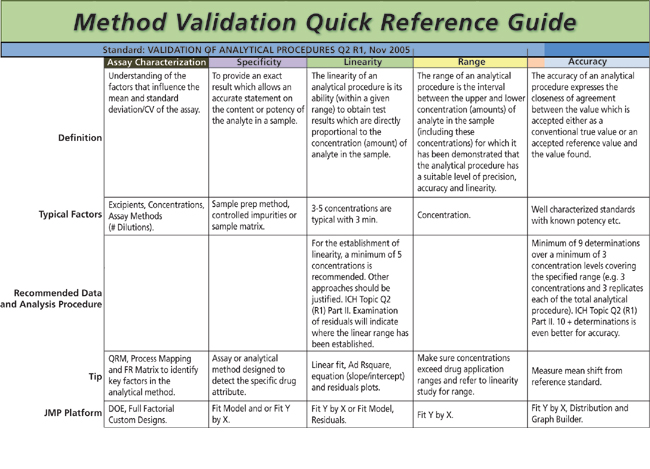
Ich q2 r1 validation of analytical procedures text and methodology.
Ich q2 r1 validation of analytical procedures.
This document does not necessarily seek to cover the testing that may be required for registration in or export to other areas of the world.
This document presents a discussion of the characteristics for consideration during the validation of the analytical procedures included as part of registration applications submitted within the ec japan and usa.
Text on validation of analytical procedures.
Q2r1 validation of analytical procedures.
To revise the ich q2r1 guideline on validation of analytical procedures.
Text and methodology this guidance has been developed by the appropriate ich expert working group and has been subject to consultation by the regulatory parties in accordance with the ich process.
The new guideline is proposed for harmonising the scientific approaches of analytical procedure development and providing the principles relating to the description of analytical procedure development process.
41 analytical procedures and methods validation before conduct of phase two and three studies are 42 discussed in the fda guidances for industry on inds for phase 2 and 3 studies of drugs.
Text and methodology note.
In november 2005 the ich incorporated q2b on methodology with the parent guidance q2a and retitled the combined document q2.
Validation of analytical procedures.
Methodology ich harmonised tripartite guideline introduction this guideline is complementary to the parent guideline which presents a discussion of the characteristics that should be considered during the validation of analytical procedures.
Text and methodology this document discusses the characteristics for consideration during the validation of the analytical procedures included as part of registration applications submitted within the ec japan and usa.
The expert working group should potentially determine the feasibility to combine both documents into one for simplification and clarity.
Health canada is pleased to announce the adoption of the ich guidance q2r1.
26 october 1993 q2 q2a approval by the steering committee under step 4and recommendation for adoption to the three ich regulatory bodies.
The page is under construction.
Validation of analytical procedures.
Ich official web site.
Furthermore this text presentation serves as a collection of terms and their definitions and is not intended to provide direction on how.
É sobre isso que podemos compartilhar ich q2 r1 validation of analytical procedures text and methodology. O administrador do blog de Texto Exemplo 29 January 2022 também coleta outras imagens relacionadas ao ich q2 r1 validation of analytical procedures text and methodology abaixo.
Essa é a discussão que podemos fornecer sobre ich q2 r1 validation of analytical procedures text and methodology. Obrigado por visitar o blog Texto Exemplo 29 January 2022.


-1.jpg)